BENEFITS OF PHOSPHOLIPIDS IN AQUAFEED DEVELOPMENT: A REVIEW
DOI:
https://doi.org/10.46754/ps.2024.01.002Keywords:
Aquafeed, Lecithin, Hydrolysed lecithin, Emulsifier, Digestibility GrowthAbstract
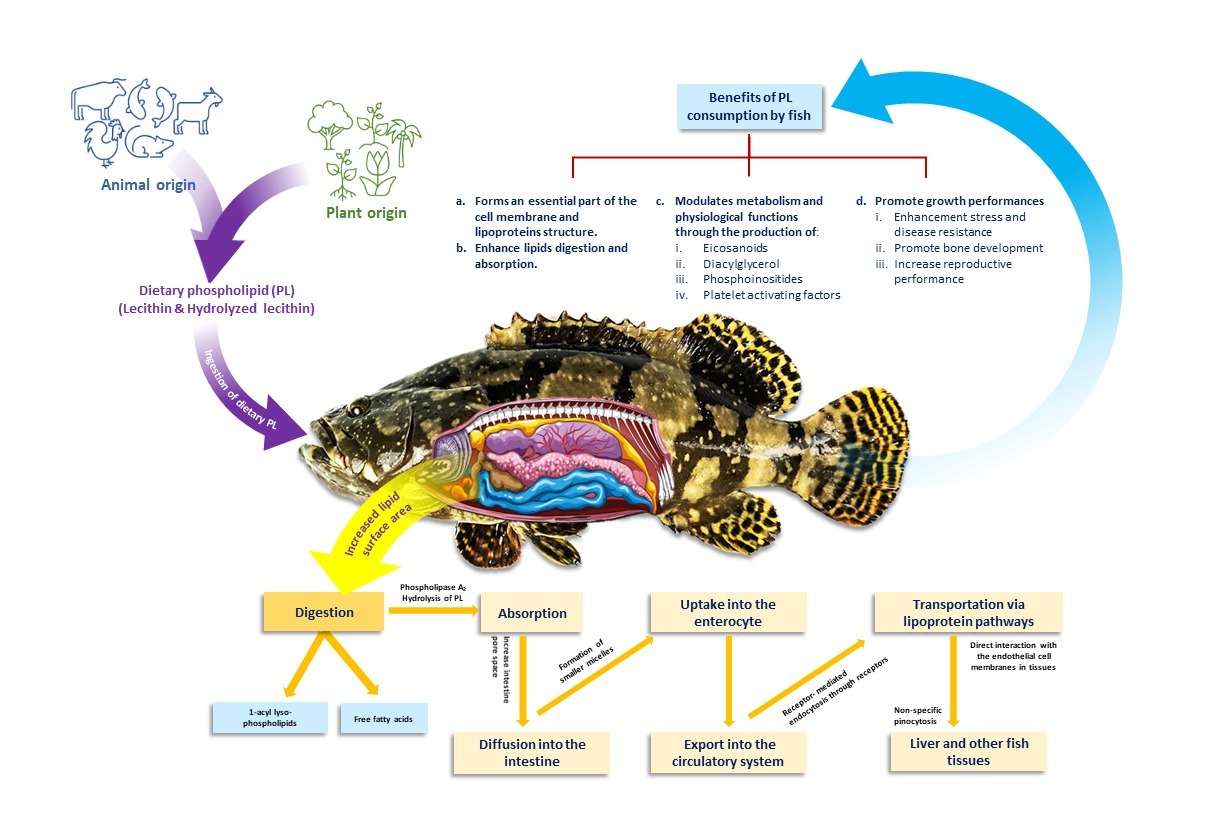
Fish oil (FO) is the main source of lipids in aquafeed, but its use has become very unsustainable due to over-exploitation, scarcity and high cost. Plant oil has been proposed as an alternative to FO, but they are less digestible and not rich in fatty acids. In addition, larval and juvenile fish are unable to synthesise sufficient phospholipids (PLs) for their metabolic need. Hence, the necessity to supplement PLs in their diets. This review describes the application and beneficial impact of dietary PLs in aquafeed. PLs are an essential component of aquafeed as they supply energy for metabolic activities and enhance digestion and absorption of other dietary lipids. Plantbased PLs such as soy lecithin serve as an emulsifier that helps lipid catabolism by facilitating enzymatic hydrolysis in the fish’s digestive system, besides improving nutrient absorption, growth and health. Studies on farm animals have confirmed the positive effects of PLs. Although the literature on aquafeed application is limited, to growth and health of farmed fish and crustaceans. The use of PLs in aquaculture is set to increase as both feed producers and farmers seek to maximise production through efficient feed utilisation and ensure sustainability in delivering quality fish to consumers.
References
Adams, S. M., Ham, K. D., Greeley, M. S., LeHew, R. F., Hinton, D. E., & Saylor, C.F. (1996). Downstream gradients in bioindicator responses: Point source contaminant effects on fish health. Canadian Journal of Fisheries and Aquatic Sciences, 53, 2177-2187. https://doi.org/10.1139/f96191 DOI: https://doi.org/10.1139/f96-191
Adel, M., Gholaghaie, M., Khanjany, P., & Citarasu, T. (2017). Effect of dietary soybean lecithin on growth parameters, digestive enzyme activity, antioxidative status and mucosal immune responses of common carp (Cyprinus carpio). Aquaculture Nutrition, 23, 1145-1152. DOI: https://doi.org/10.1111/anu.12483
Adhami, B., Amirkolaei, A. K., Oraji, H., Kazemifard, M., & Mahjoub, S. (2021a). Effects of lysophospholipid on rainbow trout (Oncorhynchus mykiss) growth, biochemical indices, nutrient digestibility and liver histomorphometry when fed fat powder diet. Aquaculture Nutrition, 27, 1779-1788. DOI: https://doi.org/10.1111/anu.13315
Arslan, M., Rinchard, J., Dabrowski, K., & Portella, M. C. (2008). Effects of different dietary lipid sources on the survival, growth, and fatty acid composition of South American catfish, Pseudoplatystoma fasciatum, surubim, juveniles. Journal of World Aquaculture Society, 39, 5161. https://doi.org/10.1111/j.17497345.2007.00133.x DOI: https://doi.org/10.1111/j.1749-7345.2007.00133.x
Balfry, S. K., & Higgs, D. A. (2001). Influence of dietary lipid composition on the immune system and disease resistance of finfish. In Lim, C., & Webster, C. D. (Eds.), Nutrition and fish health (pp.213-234). New York: The Haworth Press Inc.
Balito-Liboon, J. S., Ferdinand, R., Traifalgar, M., Pagapulan, M. J. B. B., Mameloco, E. J. G., Temario, E. E., & Corre Jr, V. L. (2018). Dietary soybean lecithin enhances growth performance, feed utilization efficiency and body composition of early juvenile Milkfish, Chanos chanos. The Israeli Journal of Aquaculture – Bamidgeh, 70, 1-9. https://evols.library.manoa.hawaii. edu/items/579f5cb0-54cf-4071-94787824ed488e4a DOI: https://doi.org/10.46989/001c.20927
Bingkun, Z., Li, H., Dongqin, Z., Yuming, G., & Adriana, B. (2011). Effect of fat type and lysophosphatidylcholine addition to broiler diets on performance, apparent digestibility of fatty acids and apparent metabolisable energy content. Animal Feed Science and Technology, 163(2-4), 177-184. https://doi. org/10.1016/j.anifeedsci.2010.10.004 DOI: https://doi.org/10.1016/j.anifeedsci.2010.10.004
Boontiam, W., Jung, B., & Kim, Y. Y. (2017). Effects of lysophospholipid supplementation to lower nutrient diets on growth performance, intestinal morphology, and blood metabolites in broiler chickens. Poultry Science, 96(3), 593-601. https://doi.org/10.3382/ps/pew269 DOI: https://doi.org/10.3382/ps/pew269
Bruning, B. A., (2009). Collective short wavelength dynamics in composite phospholipid model membranes with inelastic neutron scattering. [Doctoral’s Thesis. Georg-August-Universit¨, Gottingen].
Caballero, M. J., Izquierdo, M. S., Kjørsvik, E., Fernandez, A. J., & Rosenlund, G. (2004). Histological alterations in the liver of sea bream, Sparus aurata L., caused by short-or long-term feeding with vegetable oils. Recovery of normal morphology after feeding fish oil as the sole lipid source. Journal of Fish Diseases, 27(9), 531-541. https://doi.org/10.1111/j.13652761.2004.00572.x DOI: https://doi.org/10.1111/j.1365-2761.2004.00572.x
Campos, I., Matos, E., Maia, M. R., Marques, A., & Valente, L. M. (2019). Partial and total replacement of fish oil by poultry fat in diets for European seabass (Dicentrarchus labrax) juveniles: Effects on nutrient utilization, growth performance, tissue composition and lipid metabolism. Aquaculture, 502, 107-120. https://doi.org/10.1016/j. aquaculture.2018.12.004 DOI: https://doi.org/10.1016/j.aquaculture.2018.12.004
Chapman, M. J., Goldstein, S., Mills, G. L., & Leger C. (1978). Distribution and characterization of the serum lipoproteins and their apoproteins in the rainbow trout (Salmo gairdneri). Biochemistry, 17, 44554464. https://doi.org/10.1021/bi00614a015 DOI: https://doi.org/10.1021/bi00614a015
Du, Z. Y., Clouet, P., Huang, L. M., Degrace, P., Zheng, W. H., He, J. G., & Liu, Y. J., (2008). Utilization of different dietary lipid sources at high level in herbivorous grass carp (Ctenopharyngodon idella): Mechanism related to hepatic fatty acid oxidation. Aquaculture Nutrition, 14, 77-92. DOI: https://doi.org/10.1111/j.1365-2095.2007.00507.x
El-Bacha, T., & Torres, A. G. (2016). PLs: Physiology. In: Benjamin C, Paul, M. F, Fidel, T. (Eds.), Encyclopedia of food and health (pp. 352-359). Academic Press. DOI: https://doi.org/10.1016/B978-0-12-384947-2.00540-7
El-Sayed, A. F. M., Tammam, M. S., & Makled, S. O. (2021). Lecithin-containing bioemulsifier boosts growth performance, feed digestion and absorption and immune response of adult Nile tilapia (Oreochromis niloticus). Aquaculture Nutrition, 27, 757770. DOI: https://doi.org/10.1111/anu.13221
Furné, M., García-Gallego, M., Hidalgo, M. C., Morales, A. E., Domezain, A., Domezain, J., & Sanz, A. (2008). Effect of starvation and refeeding on digestive enzyme activities in sturgeon (Acipenser naccarii) and trout (Oncorhynchus mykiss). Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology, 149(4), 420-425. https://doi.org/10.1016/j. cbpa.2008.02.002 DOI: https://doi.org/10.1016/j.cbpa.2008.02.002
Furne, M., Hidalgo, M. C., Lopez, A., GarciaGallego, M., Morales, A. E., Domezain, A., Domezaine, J., & Sanz, A. (2005). Digestive enzyme activities in Adriatic turgeon Acipenser naccarii and rainbow trout Oncorhynchus mykiss. A comparative study. Aquaculture, 250, 391-398. DOI: https://doi.org/10.1016/j.aquaculture.2005.05.017
Gibbs, V. K., Watts, S. A., Lawrence, A. L., & Lawrence, J. M., 2009. Dietary PLs affect growth and production of juvenile sea urchin Lytechinus variegatus. Aquaculture, 292, 95103. DOI: https://doi.org/10.1016/j.aquaculture.2009.03.046
Gisbert, E., Villeneuve, L., Zambonino-Infante, J. L., Quazuguel, P., & Cahu, C. L. (2005). Dietary PLs are more efficient than neutral lipids for long-chain polyunsaturated fatty acid supply in European sea bass Dicentrarchus labrax larval development. Lipids, 40, 609–618. https://doi. org/10.1007/s11745-005-1422-0 DOI: https://doi.org/10.1007/s11745-005-1422-0
Gunstone, F. D. (2011). The world’s oils and fats. In: Turchini, G. M., Ng, W.-K., Tocher, D. R. (Eds.), Fish oil replacement and alternative lipid sources in aquaculture feeds (pp. 61–98). Boca Raton, FL: CRC Press. DOI: https://doi.org/10.1201/9781439808634-c3
Honjoh, T., Kunazawa, H., Oosaki, M., Yonemura, T., & Kashiwa, G. (1967). Effects of oxidized fish oils and added ethoxyquin on the culture of rainbow trout. Japan Oil Chemists Society, 16, 135–137. DOI: https://doi.org/10.5650/jos1956.16.135
Hosseini, S. M., Nourmohammadi, R., Nazarizadeh, H., & Latshaw, J. D. (2018). Effects of lysolecithin and xylanase supplementation on the growth performance, nutrient digestibility and lipogenic gene expression in broilers fed low‐energy wheat‐based diets. Journal of Animal Physiology and Animal Nutrition, 102(6), 1564–1573. https://doi.org/10.1111/jpn.12966 DOI: https://doi.org/10.1111/jpn.12966
Hou, Y., Yuan, Y., Lu, Y., Ma, H., Sun, P., Liang, X., Huo, Y., & Zhou, Q. (2016). Dietary soy lecithin requirement of the juvenile swimming crab (Portunus trituberculatus). Journal of Fishery Sciences of China, 40, 1753-1764.
Hu, Y., Tan, B., Mai, K., Ai, Q., Zhang, L., & Zheng, S. (2011). Effects of dietary menhaden oil, soybean oil and soybean lecithin oil at different ratios on growth, body composition and blood chemistry of juvenile Litopenaeus vannamei. Aquaculture International, 19, 459-473. DOI: https://doi.org/10.1007/s10499-010-9361-4
Huang, Y., Xu, J., Sheng, Z., Chen, N., & Li, S. (2021). Integrated response of growth performance, fatty acid composition, antioxidant responses and lipid metabolism to dietary PLs in hybrid grouper (Epinephelus fuscoguttatus♀× E. lanceolatus♂) larvae. Aquaculture, 541, 736728. DOI: https://doi.org/10.1016/j.aquaculture.2021.736728
Jafari, F., Noori, F., Agh, N., Estevez, A., Ghasemi, A., Alcaraz, C., & Gisbert, E. (2021). PLs improve the performance, physiological, antioxidative responses and, lpl and igf1 gene expressions in juvenile stellate sturgeon (Acipenser stellatus). Aquaculture, 541, 736809. https://doi. org/10.1016/j.aquaculture.2021.736809 DOI: https://doi.org/10.1016/j.aquaculture.2021.736809
Jamali, H., Ahmadifard, N., Noori, F., Gisbert, E., Estevez, A., & Agh, N. (2019). Lecithin-enriched Artemia combined with inert diet and its effects on reproduction and digestive enzymes of Aequidens rivulatus. Aquaculture, 511, 734253. DOI: https://doi.org/10.1016/j.aquaculture.2019.734253
Jaxion-Harm, J. (2021). Effects of dietary PLs on early stage Atlantic Salmon (Salmo salar) performance: A comparison among phospholipid sources. Aquaculture, 544, 737055. DOI: https://doi.org/10.1016/j.aquaculture.2021.737055
Joshi, A., Paratkar, S. G., & Thorat, B. N. (2010). Modification of lecithin by physical, chemical and enzymatic methods. European Journal Lipid Science & Technology, 108, 363-373. ejlt.200600016 https://doi.org/10.1002/ DOI: https://doi.org/10.1002/ejlt.200600016
Kanazawa, A. (1993). Essential PLs of fish and crustaceans. In Kaushik, S.J., Luquet, P. (Eds.), Fish nutrition in practice: IV international symposium on fish nutrition and feeding (pp. 519-530). INRA, France: National Institute for Agricultural Research.
Kasper, C. S., & Brown, P. B., (2003). Growth improved in juvenile Nile tilapia fed phosphatidylcholine. North America Journal of Aquaculture, 65, 39-43. https://doi. org/10.1577/1548-8454(2003)065%3C003 9:GIIJNT%3E2.0.CO;2 DOI: https://doi.org/10.1577/1548-8454(2003)065<0039:GIIJNT>2.0.CO;2
Khan, H. I., Madhubabu, E. P., Jannathulla, R., Ambasankar, K., & Dayal, J. S. (2018a). Effect of partial replacement of marine protein and oil sources in presence of lysolecithin in the diet of tiger shrimp Penaeus monodon Fabricius, 1978. Indian Journal of Fisheries, 65, 100-107. DOI: https://doi.org/10.21077/ijf.2018.65.2.74154-12
Khan, H. I., Dayal, J. S., Ambasankar, K., Madhubabu, E. P., Jannathulla, R., & Rajaram, V. (2018b). Enhancing the dietary value of palm oil in the presence of lysolecithin in tiger shrimp, Penaeus monodon. Aquaculture International, 26, 509-522. DOI: https://doi.org/10.1007/s10499-017-0235-x
Kim, M. J., Hosseindoust, A. R., Choi, Y. H., Kumar, A., Jeon, S. M., Lee, S. H., Jung, B. Y., Kil, D. Y., & Chae, B. J. (2018). An evaluation of metabolizable energy content of main feed ingredients for growing pigs when adding dietary lysoPLs. Livestock Science, 210, 99-103. DOI: https://doi.org/10.1016/j.livsci.2018.01.014
Kokou, F., Vasilaki, A., Nikoloudaki, C., Sari, A.B., Karalazos, V., & Fountoulaki, E., (2021). Growth performance and fatty acid tissue profile in gilthead seabream juveniles fed with different phospholipid sources supplemented in low-fish meal diets. Aquaculture, 544, 737052. DOI: https://doi.org/10.1016/j.aquaculture.2021.737052
Kontara, E. K. M., Djunaidah, I. S., Coutteau, P., & Sorgeloos, P. (1998). Comparison of native, lyso and hydrogenated soybean phosphatidylcholine as phospholipid source in the diet of postlarval Penaeus japonicus bate. Archives of Animal Nutrition, 51, 1-19. DOI: https://doi.org/10.1080/17450399809381901
Koven, W. M., Kolkovski S., Tandler, A., Kissil, G. W., & Sklan, D. (1993). The effect of dietary lecithin and lipase, as a function of age, on n-9 fatty acid incorporation in the tissue lipids of Sparus aurata larvae. Fish Physiology and Biochemistry, 10(5), 357364. https://doi.org/10.1007/BF00004502 DOI: https://doi.org/10.1007/BF00004502
Lall, S. P. (2002). The minerals. In J. E. Halver, R. W. Hardy (Eds.), Fish nutrition (3rd ed., pp. 259-308), San Diego: Academic Press. DOI: https://doi.org/10.1016/B978-012319652-1/50006-9
Li, X. Y., Wang J. T., Han T., Hu S. X., Jiang Y.D., & Wang C. L. (2014). Effect of dietary PLs levels and sources on growth performance, fatty acid composition of the juvenile swimming crab, Portunus trituberculatus. Aquaculture, 430, 166-172. DOI: https://doi.org/10.1016/j.aquaculture.2014.03.037
Li, B., Li, Z., Sun, Y., Wang, S., Huang, B., & Wang, J. (2019). Effects of dietary lysolecithin (LPC) on growth, apparent digestibility of nutrient and lipid metabolism in juvenile turbot Scophthalmus maximus L. Aquaculture and Fisheries, 4(2), 61-66.https://doi. org/10.1016/j.aaf.2018.11.003 DOI: https://doi.org/10.1016/j.aaf.2018.11.003
Li, H. T., Tian, L. X., Wang, Y. D., & Hu, Y. H. (2010b). Effects of lysolecithin on growth performance, body composition and hematological indices of hybrid tilapia (Oreochromis aureus × Oreochromis niloticus). Journal of Dalian Ocean University, 25(2), 143-146 (In Chinese with English abstract). https://xuebao.dlou.edu. cn/EN/abstract/abstract3400.shtml
Li, H. X., Liu, W. B., Li, X. F., Wang, J. J., Liu, B., & Xie, J. (2010a). Effects of dietary choline-chloride, betaine and lysoPLs on the growth performance, fat metabolism and blood indices of crucian carp (Carassais auratus gibelio). Journal of Fisheries of China, 34, 292-299 (In Chinese with English abstract). DOI: https://doi.org/10.3724/SP.J.1231.2010.06416
Li, S., Luo, X., Liao, Z., Liang, M., Xu, H., Mai, K., & Zhang, Y. (2022). Effects of Lysophosphatidylcholine on Intestinal Health of Turbot Fed High-Lipid Diets. Nutrients, 14, 4398. DOI: https://doi.org/10.3390/nu14204398
Li, X. F., Liu, W. B., Lu, K. L., Xu, W. N., & Wang, Y. (2012). Dietary carbohydrate/ lipid ratios affect stress, oxidative status and non-specific immune responses f f ingerling blunt snout bream, Megalobrama amblycephala. Fish Shellfish Immunology, 33, 316-323. DOI: https://doi.org/10.1016/j.fsi.2012.05.007
Liu, G., Ma, S., Chen, F., Gao, W., Zhang, W., & Mai, K. (2020). Effects of dietary lysolecithin on growth performance, feed utilization, intestinal morphology and metabolic responses of channel catfish (Ictalurus punctatus). Aquaculture Nutrition, 26, 456465. https://doi.org/10.1111/anu.13008 DOI: https://doi.org/10.1111/anu.13008
Longmuir, L. T. (2002). Lecithin. In Hubbard, A. T. (Ed.), Encyclopedia of surface and colloid science (3rd ed., pp. 2997-3006). New York, USA: Marcel Dekker Inc.
Lu, K. L., Xu, W. N., Li, X. F., Liu, W. B., Wang, L. N., & Zhang, C. N. (2013). Hepatic triacylglycerol secretion, lipid transport and tissue lipid uptake in blunt snout bream (Megalobrama amblycephala) fed high-fat diet. Aquaculture, 408, 160-168. DOI: https://doi.org/10.1016/j.aquaculture.2013.06.003
Lu, Z., Yao, C., Tan, B., Dong, X., Yang, Q., Liu, H., Zhang, S., & Chi, S. (2022). Effects of Lysophospholipid supplementation in feed with low protein or lipid on growth performance, lipid metabolism, and intestinal flora of largemouth bass (Micropterus salmoides). Nutrition, 2022, 1-12. Aquaculture https://doi. org/10.1155/2022/4347466 DOI: https://doi.org/10.1155/2022/4347466
Maldonado-Valderrama, J., Wilde, P., Macierzanka, A., & Mackie, A. (2011). The role of bile salts in digestion. Advances in Colloid and Interface Science, 165(1), 36-46. https://doi. org/10.1016/j.cis.2010.12.002 DOI: https://doi.org/10.1016/j.cis.2010.12.002
Melegy, T., Khaled, N. F., El-Bana, R., & Abdellatif, H. (2010). Dietary fortification of a natural biosurfactant, lysolecithin in broiler. African Journal of Agricultural Research, 5(21), 2886-2892.
McClements, D. J., & Gumus, C. E. (2016). Natural emulsifiers-Biosurfactants, PLs, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Advances in Colloid and Interface Science, 234, 3-26. DOI: https://doi.org/10.1016/j.cis.2016.03.002
Mohammadigheisar, M., Kim, H. S., & Kim, I. H. (2018). Effect of inclusion of lysolecithin or multi-enzyme in low energy diet of broiler chickens. Journal of Applied Animal Research, 46(1), 1198-1201. http://dx.doi.or g/10.1080/09712119.2018.1484358 DOI: https://doi.org/10.1080/09712119.2018.1484358
Morais, S., Caballero, M. J., Conceiçao, L. E., Izquierdo, M. S., & Dinis, M. T. (2006). Dietary neutral lipid level and source in Senegalese sole (Solea senegalensis) larvae: Effect on growth, lipid metabolism and digestive capacity. Comparative Biochemistry and Physiology Part B: Biochemistry & Molecular Biology, 144(1), 57-69. https://doi.org/10.1016/j. cbpb.2006.01.015 DOI: https://doi.org/10.1016/j.cbpb.2006.01.015
Mu, H., Shen, H. H., Liu, J. H., Xie, F. L., Zhang, W. B., & Mai, K. S. (2018). High level of dietary soybean oil depresses the growth and anti-oxidative capacity and induces inflammatory response in large yellow croaker Larimichthys crocea. Fish & Shellfish Immunology, 77, 465-473. https:// doi.org/10.1016/j.fsi.2018.04.017. DOI: https://doi.org/10.1016/j.fsi.2018.04.017
Niu, J., Liu, Y. J., Lin, H. Z., Mai, K. S., Yang, H. J., Liang, G. Y., & Tian, L. X. (2011a). Effects of dietary chitosan on growth, survival and stress tolerance of postlarval shrimp, Litopenaeus vannamei. Aquaculture Nutrition, 17(2), e406-e412. DOI: https://doi.org/10.1111/j.1365-2095.2010.00775.x
Niu, J., Liu, Y. J., Tian, L. X., Mai, K. S., Lin, H. Z., Chen, X., Yang, H. J., & Liang, G. Y. (2011b). Influence of dietary PLs level on growth performance, body composition and lipid class of early post larval Litopenaeus vannamei. Aquaculture Nutrition, 17, 615621. DOI: https://doi.org/10.1111/j.1365-2095.2010.00807.x
Olsen, R. E., Myklebust, R., Kaino, T., & Ringø, E. (1999). Lipid digestibility and ultrastructural changes in the enterocytes of Arctic char (Salvelinus alpinus L.) fed linseed oil and soybean lecithin. Fish Physiology and Biochemistry, 21, 35-44. https://doi.org/10.1023/A:1007726615889 DOI: https://doi.org/10.1023/A:1007726615889
Perez-Casanova, J. C., Murray, H. M., Gallant, J. W., Ross, N. W., Douglas, S. E. and Johnson, S. C. (2006). Development of the digestive capacity in larvae of haddock (Melanogrammus aeglefinus) and Atlantic cod (Gadus morhua). Aquaculture, 251(24), 377-401. DOI: https://doi.org/10.1016/j.aquaculture.2005.06.007
Poston, H. A. (1990). Effect of body size on growth, survival, and chemical composition of Atlantic salmon fed soy lecithin and choline. The Progressive Fish-Culturist, 52(4), 226-230. https://doi. org/10.1577/1548-8640(1990)052%3C022 6:EOBSOG%3E2.3.CO;2 DOI: https://doi.org/10.1577/1548-8640(1990)052<0226:EOBSOG>2.3.CO;2
Rana, K. J., Siriwardena, S., & Hasan, M. R. (2009). Impact of rising feed ingredient prices on aquafeeds and aquaculture production (Fisheries and Aquaculture Technical Paper 541). Food and Agriculture Organization of the United Nations.
Reynier, M. O., Lafont, H., Crotte, C., Sauve, P., & Gerolami, A. (1985). Intestinal cholesterol uptake: Comparison between mixed micelles containing lecithin or lysolecithin. Lipids, 20, 145-150. DOI: https://doi.org/10.1007/BF02534246
Saleh, R., Betancor, M. B., Roo, J., HernandezCruz, C. M., Moyano, F. J. and Izquierdo, M. (2013). Optimum soybean lecithin contents in microdiets for gilthead seabream (S parus aurata) larvae. Aquaculture Nutrition, 19, 585-597. DOI: https://doi.org/10.1111/anu.12009
Saleh, R., Betancor, M. B., Roo, J., BenítezDorta, V., Zamorano, M. J., Bell, J. G., & Izquierdo, M. (2015). Effect of krill PLs versus soybean lecithin in microdiets for gilthead seabream (S parus aurata) larvae on molecular markers of antioxidative metabolism and bone development. Aquaculture Nutrition, 21, 474-488. DOI: https://doi.org/10.1111/anu.12177
Sargent, J. R., Tocher, D. R., & Bell, J. G. (2002). The lipids. In J. E. Halver, and R. W. Hardy (Eds.), Fish nutrition (3rd ed., pp. 181-257). SanDiego, CA: Academic Press. DOI: https://doi.org/10.1016/B978-012319652-1/50005-7
Seiliez, I., Bruant, J. S., Zambonino Infante, J. L., Kaushik, S., & Bergot, P. (2006). Effect of dietary phospholipid level on the development of gilthead sea bream (Sparus aurata) larvae fed a compound diet. Aquaculture Nutrition, 12, 372-378. DOI: https://doi.org/10.1111/j.1365-2095.2006.00436.x
Sun, N., Chen, J., Wang, D., & Lin, S. (2018). Advance in food-derived PLs: Sources, molecular species and structure as well as their biological activities. Trends in Food Science & Technology, 80, 199-211. https:// doi.org/10.1016/j.tifs.2018.08.010 DOI: https://doi.org/10.1016/j.tifs.2018.08.010
Taghavizadeh, M., Shekarabi, S. P. H., Mehrgan, M. S., & Islami, H. R. (2020). Efficacy of dietary lysoPLs (Lipidol™) on growth performance, serum immuno-biochemical parameters, and the expression of immune and antioxidant-related genes in rainbow trout (Oncorhynchus mykiss). Aquaculture, 525, 735315. DOI: https://doi.org/10.1016/j.aquaculture.2020.735315
Tocher, D. R. (1995). Glycerophospholipid metabolism. In Biochemistry and molecular biology of fishes (Vol. 4, pp. 119-157). DOI: https://doi.org/10.1016/S1873-0140(06)80009-3
Tocher, D. R., Bendiksen, E. Å., Campbell, P. J., & Bell, J. G. (2008). The role of PLs in nutrition and metabolism of teleost fish. Aquaculture, 280, 21-34. DOI: https://doi.org/10.1016/j.aquaculture.2008.04.034
Tocher, D. R. (2003). Metabolism and functions of lipids and fatty acids in teleost fish. Reviews in Fisheries Science, 11(2), 107184. DOI: https://doi.org/10.1080/713610925
Uyan, O., Koshio, S., Ishikawa, M., Yokoyama, S., Uyan, S., Ren, T., & Hernandez, L. H. H. (2009). The influence of dietary phospholipid level on the performances of juvenile amberjack, Seriola dumerili, fed non‐fishmeal diets. Aquaculture, Nutritions, 15, 550-557. DOI: https://doi.org/10.1111/j.1365-2095.2008.00621.x
Van Hoogevest, P., & Wendel, A. (2014). The use of natural and synthetic PLs as pharmaceutical excipients. European Journal of Lipid Science and Technology, 116(9), 1088-1107. https://doi.org/10.1002/ ejlt.201400219 DOI: https://doi.org/10.1002/ejlt.201400219
Van Nieuwenhuyzen, W. (2015). Production and utilization of natural PLs. In Polar Lipids (pp. 245-276). Elsevier. https://doi. org/10.1016/B978-1-63067-044-3.50013-3 DOI: https://doi.org/10.1016/B978-1-63067-044-3.50013-3
Wang, J. T., Song, J. Y., Li, H. T., Xiao, X. W., Sun, M. M., & Wan, W. J. (2009). Effect of emulsifier on growth performance and blood biochemical index in common carp Cyprinus carpio var. Jian. Journal of Dalian Ocean University, 24, 257-260.
Wang, S., Zhang, Y., Xie, R., Zhang, N., Zhang, H., Chen, N., & Li, S. (2022). Effects of dietary phospholipids on growth performance, fatty acid composition and lipid metabolism of early juvenile largemouth bass (Micropterus salmoides). Aquaculture Research, 53, 5628-5637. https://doi.org/10.1111/ are.16044 DOI: https://doi.org/10.1111/are.16044
Weirich, C. R., & Reigh, R. C. (2001). Dietary lipids and stress tolerance of larval fish. In Lim, C., & Webster, C. D. (Eds.), Nutrition and fish health (pp. 301-312). NY: Food Products Press.
Weng, M., Zhang, W., Zhang, Z., Tang, Y., Lai, W., Dan, Z., Liu, Y., Zheng, J., Gao, S., Mai, K., & Ai, Q. (2022). Effects of dietary lysolecithin on growth performance, serum biochemical indexes, antioxidant capacity, lipid metabolism and inflammation-related genes expression of juvenile large yellow croaker (Larimichthys crocea). Fish Shellfish Immunology, 128, 50-59. DOI: https://doi.org/10.1016/j.fsi.2022.07.020
Xiao, W., Jiang, W., Feng, L., Liu, Y., Wu, P., Jiang, J., Zhang, Y., & Zhou, X. (2019). Effect of dietary enzyme-treated soy protein on the immunity and antioxidant status in the intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture Research, 50, 1411-1421. DOI: https://doi.org/10.1111/are.14016
Xie Meizhen. (2019). Phospholipids. In Laurence Melton, Fereidoon Shahidi, Peter Varelis. (Eds.), Encyclopedia of food chemistry (pp. 214-217). Academic Press. DOI: https://doi.org/10.1016/B978-0-08-100596-5.21597-7
Xu, H., Luo, X., Bi, Q., Wang, Z., Meng, X., Liu, J., Duan, M., Wei, Y., & Liang, M. (2022). Effects of dietary lysophosphatidylcholine on growth performance and lipid metabolism of juvenile turbot. Aquaculture Nutrition, 2022, 3515101. https://doi. org/10.1155/2022/3515101 DOI: https://doi.org/10.1155/2022/3515101
Zampiga, M., Meluzzi, A., & Sirri, F. (2016). Effect of dietary supplementation of lysoPLs on productive performance, nutrient digestibility and carcass quality traits of broiler chickens. Italian Journal of Animal Science, 15(3), 521-528. https://doi.org/10.1 080/1828051X.2016.1192965 DOI: https://doi.org/10.1080/1828051X.2016.1192965
Zhang, W., Wang, F., Tan, B., Dong, X., Zhang, H., Chi, S., Liu, H., Zhang, S., & Yang, Q. (2019). Effect of the dietary phosphatidylcholine at different growth stages of Pacific white shrimps, Litopenaeus vannamei. Aquaculture Nutrition, 25, 555566. DOI: https://doi.org/10.1111/anu.12857
Zhao, J., Ai, Q., Mai, K., Zuo, R., & Luo, Y. (2013). Effects of dietary PLs on survival, growth, digestive enzymes and stress resistance of large yellow croaker, Larmichthys crocea larvae. Aquaculture, 410, 122-128. DOI: https://doi.org/10.1016/j.aquaculture.2013.05.018
Zhao, P. Y., & Kim, I. H. (2017). Effect of diets with different energy and lysoPLs levels on performance, nutrient metabolism, and body composition in broilers. Poultry Science, 96(5), 1341-1347. https://doi.org/10.3382/ ps/pew469 DOI: https://doi.org/10.3382/ps/pew469
Zhou, D., Rakariyatham, K. (2019). Phospholipids. In Laurence Melton, Fereidoon Shahidi, Peter Varelis. (Eds.), Encyclopedia of food chemistry (pp. 546-549). Academic Press DOI: https://doi.org/10.1016/B978-0-08-100596-5.22357-3
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Planetary Sustainability

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.




